Genetic Factors in Fatty Liver Disease (FLD)
Abstract
Fatty Liver Disease (FLD), particularly Non-Alcoholic Fatty Liver Disease (NAFLD), represents a major global health burden with rising prevalence parallel to obesity and metabolic syndrome. While lifestyle and environmental factors are critical drivers, robust evidence demonstrates that genetic susceptibility plays a central role in disease initiation, progression, and outcomes. This document provides an expanded, scientifically rigorous, and medically oriented review of fatty liver disease with a strong emphasis on genetic determinants, molecular mechanisms, gene–environment interactions, and clinical implications. The discussion integrates insights from human genetics, molecular biology, epidemiology, and translational medicine, approaching a comprehensive academic-level review suitable for medical students, postgraduate learners, and researchers.
1. Introduction
Fatty Liver Disease is defined by excessive lipid accumulation within hepatocytes, exceeding 5% of liver weight. NAFLD has emerged as the most prevalent chronic liver disease worldwide, affecting approximately 25–30% of the global population. Importantly, only a subset of individuals with metabolic risk factors develop progressive liver disease, indicating that host-specific genetic factors significantly modulate susceptibility and disease trajectory.
Genetic research over the last two decades has shifted NAFLD from being considered purely a lifestyle-related disorder to a complex, heritable, polygenic disease. Genome-wide association studies (GWAS), candidate gene analyses, and familial studies have identified multiple loci influencing hepatic lipid metabolism, inflammation, fibrogenesis, and carcinogenesis.
2. Classification of Fatty Liver Disease
2.1 Alcohol-Related Fatty Liver Disease (AFLD)
AFLD results from chronic alcohol consumption leading to hepatic steatosis, oxidative stress, and inflammatory injury. Genetic polymorphisms (e.g., PNPLA3) also influence susceptibility and progression in alcohol-related disease, highlighting shared genetic pathways with NAFLD.
2.2 Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is diagnosed when hepatic steatosis occurs in the absence of significant alcohol intake or other secondary causes. Recently, the term Metabolic Dysfunction–Associated Steatotic Liver Disease (MASLD) has been proposed, reflecting its metabolic roots, though genetic principles remain unchanged.
3. Pathological Spectrum of NAFLD
- Simple Steatosis (NAFL) – lipid accumulation without inflammation.
- Non-Alcoholic Steatohepatitis (NASH) – steatosis with hepatocellular ballooning, inflammation, and injury.
- Fibrosis – excessive extracellular matrix deposition.
- Cirrhosis – architectural distortion and portal hypertension.
- Hepatocellular Carcinoma (HCC) – malignant transformation, sometimes without cirrhosis.
Genetic determinants influence not only disease onset but also each stage of progression.
4. Heritability and Genetic Evidence
4.1 Familial Aggregation Studies
NAFLD demonstrates strong familial clustering. First-degree relatives of affected individuals show significantly increased hepatic fat content independent of shared environmental factors.
4.2 Twin Studies
Twin studies estimate heritability of liver fat content between 20–70%. Monozygotic twins display markedly higher concordance rates for NAFLD compared to dizygotic twins, reinforcing a strong genetic component.
5. Molecular Pathogenesis of Hepatic Steatosis
Hepatic fat accumulation arises from an imbalance between:
- Fatty acid uptake
- De novo lipogenesis
- Fatty acid oxidation
- Very-low-density lipoprotein (VLDL) secretion
Genetic variants disrupt these pathways at multiple levels, predisposing hepatocytes to lipid overload.
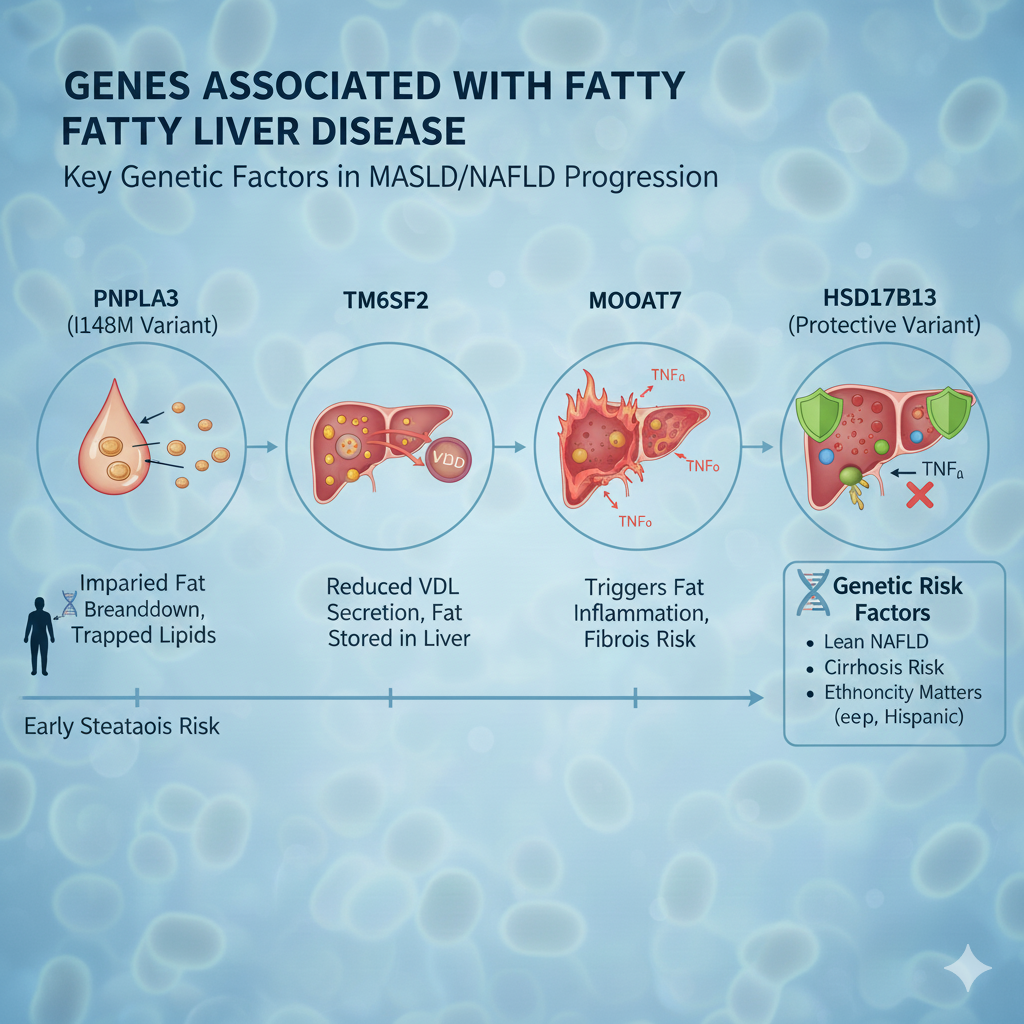
6. Major Genetic Variants Implicated in Fatty Liver Disease
6.1 PNPLA3 (Patatin-Like Phospholipase Domain-Containing Protein 3)
The PNPLA3 I148M variant is the strongest known genetic risk factor for NAFLD and NASH.
Mechanisms:
- Impaired triglyceride hydrolysis
- Lipid droplet accumulation
- Altered retinol metabolism in hepatic stellate cells
Clinical Impact:
- Increased steatosis
- Accelerated fibrosis
- Higher risk of hepatocellular carcinoma
6.2 TM6SF2 (Transmembrane 6 Superfamily Member 2)
TM6SF2 regulates VLDL secretion. Loss-of-function variants reduce lipid export, causing hepatic lipid retention.
Paradoxical Effect: Reduced circulating lipids but increased liver injury risk.
6.3 MBOAT7
MBOAT7 participates in phosphatidylinositol remodeling. Reduced activity increases inflammatory signaling and fibrogenesis.
6.4 GCKR (Glucokinase Regulatory Protein)
GCKR variants increase glycolytic flux and lipogenesis, linking carbohydrate metabolism directly to hepatic fat accumulation.
6.5 HSD17B13
Protective variants of HSD17B13 reduce inflammation and fibrosis, demonstrating that some genetic factors mitigate disease severity.
7. Polygenic Risk and Genetic Architecture
NAFLD is a polygenic disorder involving dozens of loci with small effect sizes. Polygenic risk scores (PRS) combine multiple variants to estimate individual disease risk and progression probability.
8. Gene–Environment Interactions
Genetic predisposition is amplified or mitigated by environmental exposures:
- High-fructose diets
- Saturated fat intake
- Sedentary behavior
- Alcohol use
Individuals carrying high-risk alleles show exaggerated responses to obesogenic environments.
9. Epigenetic Regulation in Fatty Liver Disease
Epigenetic modifications regulate gene expression without altering DNA sequence.
9.1 DNA Methylation
Aberrant methylation patterns influence lipid metabolism genes.
9.2 Histone Modifications
Histone acetylation alters chromatin accessibility, affecting inflammatory gene transcription.
9.3 MicroRNAs
Specific microRNAs (e.g., miR-122) regulate lipid homeostasis and hepatocyte integrity.
10. Role of Mitochondrial Genetics
Mitochondrial dysfunction contributes to oxidative stress and impaired β-oxidation. Variations in mitochondrial DNA and nuclear-encoded mitochondrial genes exacerbate NAFLD progression.
11. Gut–Liver Axis and Host Genetics
Host genetics influence gut microbiota composition, intestinal permeability, and endotoxin signaling, indirectly modulating hepatic inflammation and steatosis.
12. Genetics of Fibrosis Progression
Fibrosis progression is the strongest predictor of mortality. Genetic variants modulate:
- Stellate cell activation
- Extracellular matrix deposition
- Tissue remodeling
13. Genetic Risk of Hepatocellular Carcinoma
Variants in PNPLA3 and TM6SF2 significantly increase HCC risk, even in non-cirrhotic NAFLD.
14. Clinical Implications of Genetic Insights
14.1 Risk Stratification
Genetics enables identification of high-risk individuals for closer monitoring.
14.2 Precision Medicine
Future therapies may target genotype-specific pathways.
15. Genetic Testing: Current and Future Perspectives
While not routine, genetic testing holds promise for personalized NAFLD management. Ethical, social, and economic factors must be addressed.
16. Therapeutic Implications
16.1 Lifestyle Intervention
Even genetically predisposed patients benefit significantly from weight loss and exercise.
16.2 Pharmacogenomics
Genotype-guided therapy may optimize treatment response and reduce adverse effects.
17. Emerging Research and Future Directions
- Multi-omics integration
- Gene editing approaches
- Longitudinal population studies
18. Conclusion
Fatty liver disease is a genetically complex, metabolically driven condition. Genetic susceptibility profoundly influences disease initiation, progression, and outcomes. A deeper understanding of molecular genetics is reshaping NAFLD from a lifestyle-associated disorder into a precision medicine paradigm. Continued integration of genetics into clinical practice promises improved prevention, early detection, and individualized treatment strategies.

18. Ayurvedic Perspective and Management of Fatty Liver Disease (Yakrit Roga / Yakrit Meda Vriddhi)
18.1 Concept of Fatty Liver in Ayurveda
In Ayurveda, fatty liver disease does not appear as a single named entity but is understood through the concepts of Yakrit Roga (liver disorders), Meda Vriddhi (abnormal increase of fat tissue), Ama Sanchaya (toxic metabolic by‑products), and Agnimandya (impaired digestive and metabolic fire). NAFLD most closely correlates with conditions such as Yakritodara, Santarpanajanya Vyadhi (diseases caused by over‑nutrition), and Kapha‑Meda Dushti.
Ayurveda views the liver (Yakrit) as the principal seat of Ranjaka Pitta, responsible for metabolism, blood formation, and detoxification. Impairment of Agni at both Jatharagni (digestive fire) and Dhatvagni (tissue metabolism) levels leads to improper fat metabolism and accumulation within the liver.
18.2 Ayurvedic Pathogenesis (Samprapti)
The Ayurvedic disease mechanism of fatty liver can be summarized as follows:
- Excessive intake of Guru, Snigdha, Madhura foods (heavy, oily, sweet foods)
- Sedentary lifestyle and lack of physical activity
- Kapha and Meda aggravation
- Weakening of Agni
- Formation of Ama
- Obstruction of Srotas (micro‑channels), especially Medovaha and Raktavaha Srotas
- Fat deposition and inflammation in Yakrit
This multi‑step metabolic disturbance closely parallels modern concepts of insulin resistance, dyslipidemia, oxidative stress, and chronic inflammation.
18.3 Dosha Involvement
- Kapha Dosha: Primary contributor due to its role in fat accumulation and heaviness
- Pitta Dosha: Involved in inflammatory and hepatocellular injury stages
- Vata Dosha: Becomes aggravated in advanced fibrosis and cirrhosis stages
Effective Ayurvedic treatment therefore aims at Kapha‑Meda Shamana, Agni Deepana, and Ama Pachana.
18.4 Ayurvedic Treatment Principles (Chikitsa Siddhanta)
Management of fatty liver in Ayurveda follows these core principles:
- Nidana Parivarjana – elimination of causative dietary and lifestyle factors
- Agni Deepana & Pachana – strengthening digestive and metabolic fire
- Meda Lekhana – reduction of excess fat tissue
- Yakrit Uttejaka – stimulation and protection of liver function
- Shodhana – detoxification therapies where indicated
- Rasayana – rejuvenation and prevention of progression
18.5 Classical Ayurvedic Medicines for Fatty Liver Disease
18.5.1 Single‑Herb (Eka Dravya) Therapies
- Bhumyamalaki (Phyllanthus niruri)
Hepatoprotective, anti‑inflammatory, antioxidant; widely used in Yakrit Vikara - Katuki (Picrorhiza kurroa)
Potent Tikta Rasa, improves bile flow, reduces hepatic fat - Guduchi (Tinospora cordifolia)
Immunomodulatory, anti‑oxidative, improves insulin sensitivity - Punarnava (Boerhavia diffusa)
Reduces hepatic congestion and edema, supports detoxification - Kalmegha (Andrographis paniculata)
Strong hepatoprotective and anti‑fibrotic properties
18.5.2 Classical Polyherbal Formulations
- Arogyavardhini Vati
Considered a gold‑standard formulation for fatty liver; improves lipid metabolism and liver enzymes - Liv‑52 (modern proprietary formulation)
Hepatoprotective, antioxidant, improves appetite and liver function tests - Punarnavadi Mandura
Useful in fatty liver associated with anemia and edema - Phalatrikadi Kashaya
Detoxifies liver and improves bile secretion - Triphala Guggulu
Meda‑hara, improves lipid profile and bowel regularity
18.6 Panchakarma Therapies in Fatty Liver Disease
18.6.1 Virechana (Therapeutic Purgation)
Virechana is the most important Panchakarma therapy for liver disorders. It eliminates excess Pitta and Kapha, detoxifies the liver, and improves metabolic function.
18.6.2 Basti (Medicated Enema)
Used in chronic cases and fibrosis‑prone patients to regulate Vata and improve metabolism.
18.6.3 Udvartana (Dry Powder Massage)
Promotes fat metabolism and reduces Kapha and Meda accumulation.
18.7 Ayurvedic Diet (Ahara Chikitsa)
Recommended dietary principles include:
- Light, warm, freshly prepared meals
- Bitter and astringent tastes (Tikta, Kashaya Rasa)
- Barley, millet, green gram, leafy vegetables
- Avoidance of refined sugars, fried foods, alcohol
Specific beneficial items:
- Buttermilk (Takra)
- Turmeric (Haridra)
- Garlic (Lasuna)
- Fenugreek (Methi)
18.8 Lifestyle and Yoga Therapy
- Daily physical activity
- Early sleep and regular meal timing
- Yoga asanas: Dhanurasana, Bhujangasana, Ardha Matsyendrasana
- Pranayama: Kapalabhati, Nadi Shodhana
18.9 Evidence‑Based Integration with Modern Medicine
Recent clinical studies support the hepatoprotective effects of several Ayurvedic herbs through mechanisms such as:
- Reduction of oxidative stress
- Improvement of insulin sensitivity
- Anti‑inflammatory and anti‑fibrotic effects
Integrative management combining lifestyle modification, modern diagnostics, and Ayurvedic therapy offers promising outcomes, especially in early‑stage NAFLD.
18.10 Safety, Standardization, and Limitations
- Treatment must be individualized based on Prakriti
- Long‑term unsupervised use of metallic preparations is discouraged
- Quality control and standardization are essential
19. Conclusion
Ayurveda provides a comprehensive, holistic framework for understanding and managing fatty liver disease. By addressing metabolic imbalance at its root through diet, lifestyle, herbal medicine, and detoxification, Ayurvedic therapy offers both preventive and therapeutic benefits. When appropriately integrated with modern medical care, Ayurveda may play a valuable role in slowing disease progression, improving liver function, and enhancing overall metabolic health.
Fatty liver disease is a genetically complex, metabolically driven condition. Genetic susceptibility profoundly influences disease initiation, progression, and outcomes. A deeper understanding of molecular genetics is reshaping NAFLD from a lifestyle-associated disorder into a precision medicine paradigm. Continued integration of genetics into clinical practice promises improved prevention, early detection, and individualized treatment strategies.
Internal Links (healthguideme.com )
- Diabetes & Fatty Liver connection
👉 Read more about diabetes and metabolic health - Lifestyle & metabolic disorders
👉 Learn more about lifestyle-related health conditions - Hormonal & metabolic health topics
👉 Explore sex life and overall health
Outbound External Links (Authority – DoFollow)
- World Health Organization (WHO) – Metabolic Diseases
https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases - NIH (NIDDK) – Fatty Liver Disease (NAFLD & NASH)
https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash - Mayo Clinic – Fatty Liver Disease Overview
https://www.mayoclinic.org/diseases-conditions/fatty-liver-disease/symptoms-causes/syc-20354567 - PubMed – NAFLD Research Articles
https://pubmed.ncbi.nlm.nih.gov/?term=nonalcoholic+fatty+liver+disease
Fatty liver disease is a growing global health concern linked to metabolic disorders and lifestyle factors. According to the World Health Organization (WHO), non-communicable diseases are rising worldwide, while clinical guidance from the NIH on fatty liver disease highlights the role of insulin resistance and genetics. Additional medical insights are provided by the Mayo Clinic’s fatty liver disease overview, and extensive scientific evidence can be accessed through PubMed research on NAFLD.
fatty liver disease, non alcoholic fatty liver disease, NAFLD, NASH, fatty liver disease symptoms, fatty liver disease causes, fatty liver disease treatment, how to reverse fatty liver, fatty liver diet plan, fatty liver and diabetes, insulin resistance and fatty liver, liver steatosis, hepatic steatosis, fatty liver disease stages, fatty liver disease diagnosis, fatty liver disease prevention, fatty liver disease genetics, PNPLA3 gene fatty liver, metabolic fatty liver disease, MASLD, fatty liver lifestyle changes, fatty liver exercise, fatty liver natural treatment, ayurvedic treatment for fatty liver, integrative medicine for fatty liver, fatty liver disease prognosis, fatty liver disease complications fatty liver disease treatment how to reverse fatty liver disease
fatty liver disease, non alcoholic fatty liver disease, NAFLD, fatty liver symptoms, fatty liver treatment, how to reverse fatty liver, fatty liver diet, fatty liver natural remedies, fatty liver lifestyle changes, fatty liver and diabetes, liver detox tips, liver health tips, fatty liver ayurveda, ayurvedic treatment for fatty liver, liver cleansing diet, metabolic liver disease, liver wellness, healthy liver tips